EMD receives ~$500k in non dilutive funding
Our biotech Investment Emyria (ASX: EMD) just received a ~$500K grant to fund the development of its MDMA analogue drug program.
The grant was awarded by the Future Health Research and Innovation (FHRI) Seed Fund in partnership with the University of Western Australia (UWA).
The grant means EMD get almost half a million dollars of non dilutive funding to try and prove up an MDMA analogue drug over the next ~18 months.
The grant is typically only given to company’s where the FHRI fund thinks there is “high commercial potential” so we see it as good external validation of the work EMD is doing with psychedelic therapies.
The two drug discovery programs EMD is most advanced with are for:
- The enhancement and extension of levodopa therapy for Parkinson’s disease (PD)
- The development of next-generation entactogens for drug-assisted psychotherapy
Next for these two programs will be to file patents for the MDMA analogues EMD has developed together with the UWA and then to finalise licensing discussions so that EMD has a clear pathway for commercialising the analogues.
A key reason we are Invested in EMD is because it is leading the way in a relatively novel part of the biotech space and it is a unique vertically integrated exposure to Psychedelic biotech space.
What does EMD do?
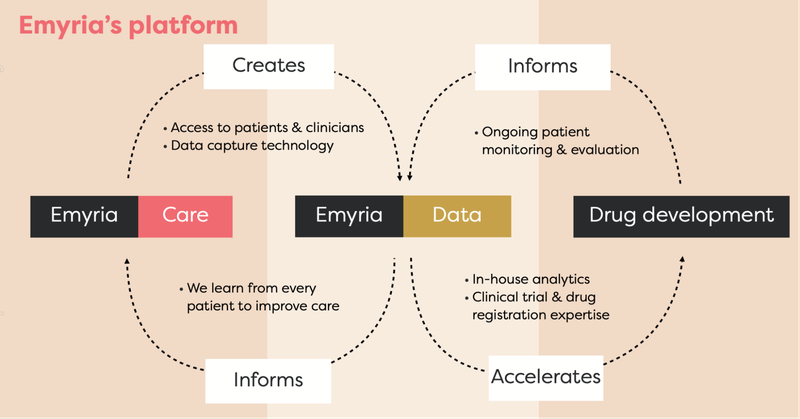
EMD is an “integrated clinical drug development and care delivery” company.
EMD’s primary area of focus is on psychedelics such MDMA, psilocybin and, more recently, ketamine.
The company is looking to use these drugs to support treatments for mental health problems- first targeting PTSD, and then expanding into other areas.
EMD has an integrated business strategy because it has:
- Treatment centres: Places where treatments can be administered with wrap-around care.
- Treatments: EMD can legally source the drugs, which include psilocybin, ketamine, MDMA and an ongoing drug development program into analogues, aiming to make the treatment work better for a range of conditions.
- Data: Through its clinics and approved clinical trials, EMD collects and owns valuable patient data to help improve its drug treatments and therapy programs.
- Clinical drug development: EMD is developing its own MDMA analogues to harness MDMA drug treatments.
One of the key reasons we are Invested in EMD is because it owns its own physical clinics which means it has the infrastructure ready to deliver any therapies that the company develops.
See our take on EMD’s business strategy here: What is EMD’s business strategy?
What’s Next for EMD?
- Finalise Funding Deal with Reach Wellness 🔄
- MDMA-AT Phase 2b patient recruitment updates 🔄
- Approval for ketamine assisted therapy protocol 🔄
- Secure key approvals for psilocybin use 🔄
- Secure a major payer partnership (we see this as a major catalyst) 🔲






